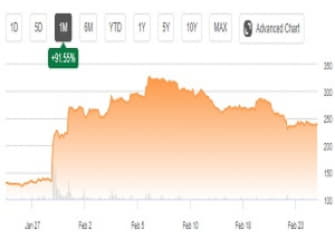
I was initially bullish on Novavax (NVAX), but the bloom fell off the rose after Pfizer (PFE) and Moderna (MRNA) were the first to receive Emergency Use Authorization for a COVID-19 vaccine. The moment of truth finally arrived for Novavax earlier this month. Clinical trials reported efficacy of 89%. This implied Novavax's vaccine could compete head to head with that of Pfizer and Moderna. The company followed that up with a deal to provide 1.1 billion COVID-19 vaccine doses to the Vaccine Alliance:
Novavax, Inc., a biotechnology company developing next-generation vaccines for serious infectious diseases, today announced a Memorandum of Understanding ("MOU") with Gavi, the Vaccine Alliance (Gavi), to provide 1.1 billion cumulative doses of NVX-CoV2373, Novavax' recombinant protein-based COVID-19 vaccine candidate, for the COVAX Facility. The vaccine doses will be manufactured and distributed globally by Novavax and Serum Institute of India ("SII"), the latter under an existing agreement between Gavi and SII. NVX-CoV2373 is being studied in two ongoing pivotal Phase 3 clinical trials: in the United States and Mexico, as well as in the United Kingdom (U.K.), for the prevention of COVID-19. Novavax has previously reported positive interim efficacy results from its U.K. trial.
READ FULL ARTICLE HERE