Summary
- NVAX recently announced it had initiated Phase 3 trials for a COVID-19 vaccine in the U.S.
- If NVAX wins FDA approval, then it could generate billions in revenue related to COVID-19. The moment of truth is here.
- Last week NVAX and MRNA both fell in the double-digit percentage range. I expect more volatility ahead. NVAX is a hold.
- This idea was discussed in more depth with members of my private investing community, Shocking The Street. Get started today »
Novavax (NVAX) recently announced it had initiated a Phase 3 study for its COVID-19 vaccine in the U.S.
Novavax announces that it has initiated the Phase 3 study for its COVID-19 vaccine candidate in the U.S. and Mexico.
The study, involving 30,000 volunteers aged 18 years and older, will evaluate the efficacy, safety, and immunogenicity of NVX-CoV2373 in a randomized, placebo-controlled, observer-blinded trial, conducted in 155 sites.
Two-third of subjects will randomly receive two intramuscular injections of the vaccine, administered 21 days apart, with one-third receiving the placebo. The company has designed the study to evaluate the vaccine candidate’s impact on a diverse population: More than 25% of subjects are 65 years old or older, and more than 15% comprises black/African American.
The U.S. trial had been delayed. Novavax faced a bevy of questions from the FDA pursuant to its partnering arrangement with Fujifilm Diosynth Technologies involving its commercial-scale manufacturing facility. Novavax had used smaller clinical-scale batches in previous trials. The fact that the Phase 3 study is now under way implies the FDA may be comfortable with the company's manufacturing process.
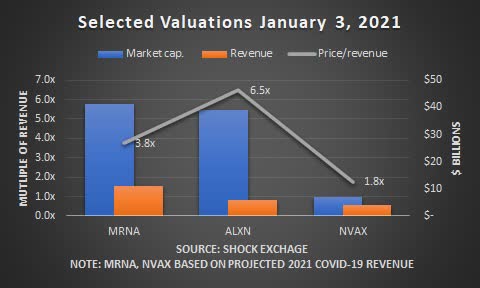
About $39 billion in COVID-19 revenue in 2021 is at stake. Pfizer (PFE), Moderna (MRNA) and AstraZeneca (NASDAQ:AZN) have each had their vaccines approved for emergency use. Bernstein analysts believe Novavax's vaccine could generate $4 billion of the $39 billion COVID-19 market this year. However, Pfizer has the poll position and Novavax is still awaiting FDA approval. The longer it takes for the company to receive FDA approval, the higher the potential its $4 billion revenue estimate could slide.