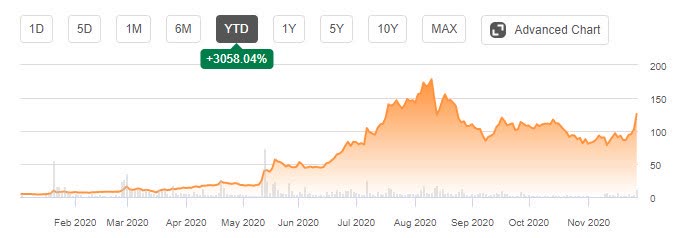
Novavax (NASDAQ:NVAX) provides an update on its COVID-19 vaccine program. NVX?CoV2373 is a stable, prefusion protein antigen derived from the genetic sequence of the SARS-CoV-2 coronavirus spike (S) protein and adjuvanted with Matrix?M.
Two of the three planned late-stage efficacy trials for NVX-CoV2373 are fully enrolled, and more than 20,000 participants have been dosed to-date.
Novavax completed enrollment of 15,000 participants in a Phase 3 U.K. trial. Interim data are expected in early Q1 2021.
These data are expected to serve as the basis for licensure application in the U.K., European Union and other countries.
The Phase 2b trial taking place in South Africa is now fully enrolled. A total of 4,422 volunteers are taking part.


































