Summary
- Novavax investors are preparing for a decisive 2020 that will contain multiple events and catalysts that will determine the fate of the company's NanoFlu and ResVax programs.
- I provide some background on these programs and discuss the importance of their 2020 pending catalysts.
- I disclose my plans for my NVAX position as we closeout 2019 and head into 2020.
Novavax (NVAX) remains focused on the company’s ResVax and NanoFlu product candidates and it appears the company has made noteworthy progress in both programs. Novavax reached an agreement with the FDA on the design of NanoFlu’s Phase III clinical trial and that it would be a pivotal trial. As for ResVax, both the EMA and the FDA have suggested that Novavax conduct a supplementary Phase III clinical trial to confirm the RSV vaccine efficacy. In addition, Novavax is in discussions with regulatory authorities and potential partners to find a way to get ResVax on the market. Together, NanoFlu and ResVax will provide multiple catalysts and events that will make 2020 a decisive year for NVAX investors.
I intend to review the NanoFlu and ResVax programs and how they will be in play during 2020. In addition, I provide my views on 2020 and how I plan to manage my NVAX position around these pending events.
NanoFlu
NanoFlu is the company’s recombinant nanoparticle technology flu vaccine that contains a safe and potent adjuvant to produce a cutting edge flu vaccine. Novavax has come to an arrangement with the FDA that the Phase III trial results will be seen as pivotal data and will be used in determining NanoFlu’s ability to be approved. This pivotal trial evaluated NanoFlu’s immunogenicity and safety in adults age 65 and older. The company enrolled 2,652 healthy patients who received either NanoFlu, or Sanofi’s (SNY) Fluzone Quadrivalent. The trial’s primary immunogenicity data will be taken on Day 28 with sera samples. The goal is to show that NanoFlu is safe and non-inferior to Fluzone Quadrivalent in HAI titers. Novavax expects to report its top-line data in the first quarter of 2020. If the trial is successful, the data should be enough to support a BLA with an accelerated approval potentially before the year-end.
ResVax
ResVax is the company’s maternal RSV vaccine to protect infants from a potentially fatal illness. The EMA and the FDA are requesting an additional Phase III trial to reaffirm the efficacy Novavax recorded in the recent Phase III trial. Novavax is in continuing discussions with both global regulatory authorities and potential partners to find a way to get ResVax on the global market.
Novavax recently presented additional safety, efficacy and safety findings that have provided me some hope that the ResVax program is not dead in the water. ResVax failed to hit its primary endpoint, however, Novavax recently presented updated data from the Prepare trial. The analysis revealed a 59.6% decrease in SAEs through the baby’s first year of life. In addition, comparable efficacy of about 50% continued over a year (Figure 1).
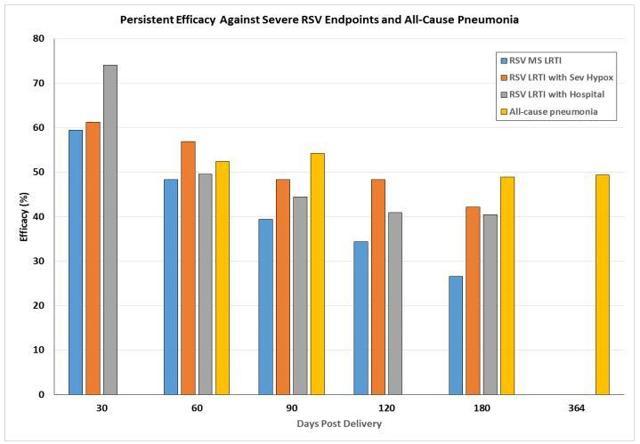
Figure 1: ResVax Efficacy Interval (Source NVAX)


































